Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Formulation and Evaluation of Phytosomes of Pterocarpus santalinus Extract
Authors: Pooja Dhadse, Jyoti Saxena
DOI Link: https://doi.org/10.22214/ijraset.2024.57964
Certificate: View Certificate
Abstract
Novel drug delivery system (NDDS) phytosomes were prepared by complexing phospholipids: Cholesterol and Leaves extract of Pterocarpus santalinus can act as molecular adhesive to bind components together. Their ability to penetrate lipid-rich biomembranes and shield the essential components of the herbal extract from being destroyed by digestive secretions and gut bacteria increases bioavailability. The bioavailability of the herbal formulation is increased by phytosomes\' ability to distribute the standardised plant extracts and phytoconstituents through different routes of drug administration methods. The present study directed toward the development and evaluation of phytosomes of Pterocarpus santalinus. Through an in-vitro dissolution study, the produced complex\'s physicochemical characteristics were examined. The outcome demonstrated that there was no formation of a novel chemical between the hydroalcoholic extract and the phospholipids in the Methanolic extract-phospholipid complex. Prepared Pterocarpus santalinus extract phytosomes showed better bioavailability.
Introduction
I. INTRODUCTION
Natural products, especially plants, have been used by humans throughout history to maintain and enhance health and to treat disease, pain, and illness1. But as traditional knowledge of these medicinal plants has become more widely known, herbal traditional drugs have begun to take the place of allopathic medicines in many cases. This is demonstrated by the increase in the number of doctors and public health specialists who are reconsidering alternatives to conventional medicine in general and traditionally plant-based drugs in particular across the globe. Today, there is a global search for plant-based medicines that are economically viable, non-toxic, and safe for use in treating a variety of ailments2-3.
Plant extract formulation into dosage forms is a complex process that cannot be seen solely as a pharmaceutical technology issue4. In order to improve the existing products' poor absorption, quick metabolism, and quick systemic clearance, researchers are developing solutions based on plant extracts. Various strategies will be used to address these issues5. The majority of a plant's biologically active components are water soluble molecules.
However, water-soluble phytoconstituents are poorly absorbed either as a result of their large molecular size, which limits passive diffusion, or as a result of their decreased lipid solubility. More than a typical liposome, phytosomes are the molecule that can penetrate lipid-rich biomembranes6.
The plant Pterocarpus santalinus (Fabaceae, also referred to as Red Sanders), is an endemic species that can only be found in the southern Eastern Ghats of India, mainly in the state of Andhra Pradesh7. Both domestically and internationally, Red Sanders' wavy-grained heartwood commands a premium. Along with its extensive use in furniture, the red dye made from the wood is also employed as a colouring additive for food, medicine, and textiles. In traditional and folkloric medicine, the wood is used to treat a wide range of issues, including diabetes, prickly heat, skin conditions, and a number of other maladies8.
In order to create Phytosomes that are lipid-compatible molecular complexes, which considerably increase their absorption and bioavailability, standardised plant extracts or water-soluble phyto components are integrated into phospholipids using a patented process called phytosomes9.
The Phytosomes preparations method, which produces a tiny cell, protects the delicate components of the herbal extract from being destroyed by digestive secretions and gut bacteria. The superior pharmacokinetic and pharmacological properties of phytosomes make them an advantageous treatment option for acute and chronic liver diseases with toxic metabolic, infectious, or degenerative causes. Along with being utilised in a variety of activities, it can also be found in pharmaceutical and cosmetic formulations10. The present objective of the study is to prepare and evaluate Pterocarpus santalinus extract loaded Phytosome and assessment of its in-vitro anti-diabetic activity.
II. METHOD
Material - Hydro-alcoholic extract of Pterocarpus santalinus leaves, phospholipids (Loba chemie Pvt. Ltd., Mumbai), Cholesterol (Loba chemie Pvt. Ltd., Mumbai), Methanol, solvents and other chemical reagents are analytical grade.
A. Preliminary Screening
- Determination of ?max of Pterocarpus santalinus extract
a. Preparation of the Sample: Take a small amount (10mg) of Pterocarpus santalinus extract and dissolve it in 0.1 N HCl to obtain a concentration of 1 mg/mL. Prepare suitable dilution to make it to a concentration range of 5-25 μg/mL. The spectrum of this solution was run in 200-800 nm range in U.V. spectrophotometer (Labindia-3000+).
b. Calibration of the Spectrophotometer: Perform a baseline correction by using the solvent as a blank and calibrate the spectrophotometer as per the manufacturer's instructions.
c. Scanning the Sample: Pour the sample solution into a cuvette and place it in the spectrophotometer. Start scanning the sample in the selected wavelength range.
d. Determination of λmax: Observe the absorption spectrum of the sample and note the wavelength at which the highest absorbance is obtained. This wavelength is the λmax of the Pterocarpus santalinus extract11.
2. Drug Excipient compatibility study by FT-IR
a. Sample Preparation: Weigh out the drug and excipient in a 1:1 ratio and mix thoroughly. Prepare the sample as a thin film by placing a small amount of the mixture between two KBr discs.
b. IR Spectroscopy: Perform FT-IR spectroscopy on the sample using a Fourier Transform Infrared spectrometer. Scan the sample in the range of 4000-400 cm-1.
c. Analysis: Analyze the FT-IR spectra obtained from the sample to identify any changes in the functional groups of the drug or excipient. Compare the spectra of extract and prepared phytosomes formulation to determine any changes that may have occurred due to the interaction between the drug and the excipient.
d. Interpretation: Interpret the results obtained from the FT-IR spectra to determine the compatibility of the drug and excipient. The presence of new peaks or the disappearance of existing peaks may indicate the formation of new chemical entities or the degradation of the drug.
3. Drug Excipient compatibility study by DSC
With the use of a differential scanning calorimeter, thermograms were captured. In flat-bottomed aluminium pans, the extract and manufactured phytosomes formulation (5–10 mg) were weighed and hermetically sealed. With alumina serving as the reference standard, these samples were heated between 50 and 400 oC at a steady rate of 100 oC per minute in an environment of nitrogen (200 ml/min).
B. Preparation of Phytosomes
Cholesterol and the leaf extract from Pterocarpus santalinus were used to prepare the complex in the following ratios: 1:0.5:1.0, 1:1:1.0, 2:0.5:1.0, and 2:0.5:1.0. A 100 ml round-bottom flask was filled with the weighted amount of extract, cholesterol, phospholipids, and as the reaction medium 25 ml of CH2Cl2 was added. For the reaction of the complex the mixture was refluxed for 3 hours while maintaining a temperature of 50°C. After the resulting clear mixture had evaporated, 20 ml of n-hexane was stirred in. To get rid of the traces of solvents, the precipitate was filtered and dried under vacuum. The residue were collected, desiccated overnight, and stored in an amber-colored glass bottle at room temperature12.
Table1: Different formulations of phytosomes
|
Formulation |
Phospholipids (%) |
Cholesterol (%) |
Extract Concentration (%) |
Dichloromethane Concentration (ml) |
|
F1 |
1 |
0.5 |
1 |
25 |
|
F2 |
1 |
1 |
1 |
25 |
|
F3 |
2 |
1.5 |
1 |
25 |
|
F4 |
2 |
0.5 |
1 |
25 |
C. Characterization
- Entrapment Efficiency
Procedure - Sample Preparation: Take a known amount of the Phytosomes and centrifuge it at a predetermined speed (usually 15,000 rpm) for a specific duration (usually 15 minutes) to separate the unentrapped drug from the Phytosomes.
Supernatant Collection: Collect the supernatant containing the unentrapped drug using a pipette and transfer it to a separate vial.
Drug Quantification: Measure the amount of drug in the supernatant using a suitable UV-Vis spectrophotometry at 420.0 nm for determination of flavanoids.
Entrapped drug quantification: Calculate the amount of drug entrapped in the Phytosomes by subtracting the amount of unentrapped drug from the initial amount of drug used to prepare the Phytosomes13.
Entrapment efficiency calculation: Calculate the entrapment efficiency of the Phytosomes using the following formula:
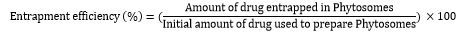
2. Particle Size and Size Distribution
Dilute the nanoparticle sample with distilled water or a suitable buffer to obtain a concentration suitable for analysis. It is important to ensure that the sample is homogeneous and free from any aggregates or debris. Measure the particle size using a Malvern Zetamaster ZEM 5002, Malvern, UK as dynamic light scattering (DLS. In DLS, the sample is illuminated with a laser and the scattered light is measured to determine the particle size14.
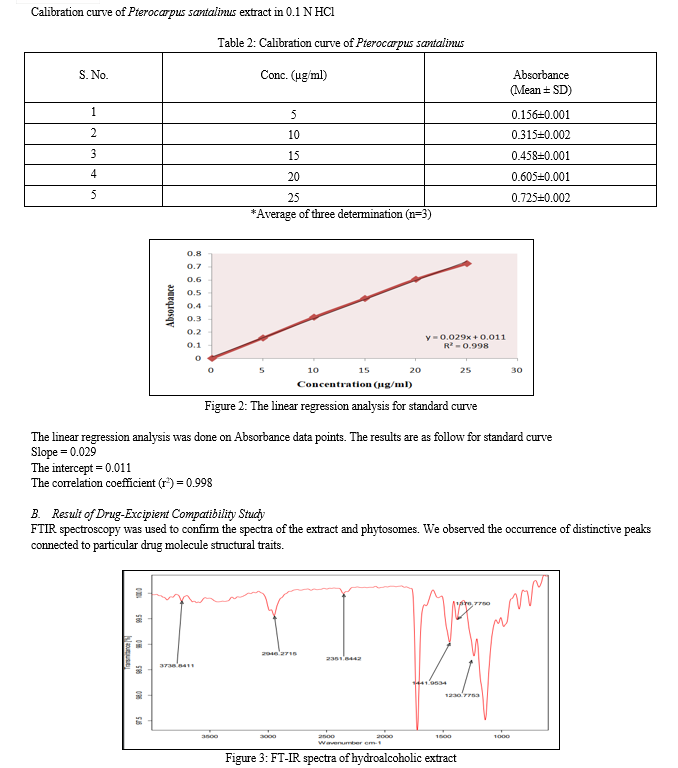
3. Transmission Electron Microscopy
TEM was used to analyse the surface morphology; a drop of the sample was placed on a copper grid that had been coated with carbon, and after 15 minutes, it was negatively stained with a 1% aqueous solution of phosphotungustic acid. The grid was fully dried by air, and samples were examined using transmission electron microscopy (TEM Hitachi, H-7500 Tokyo, Japan).
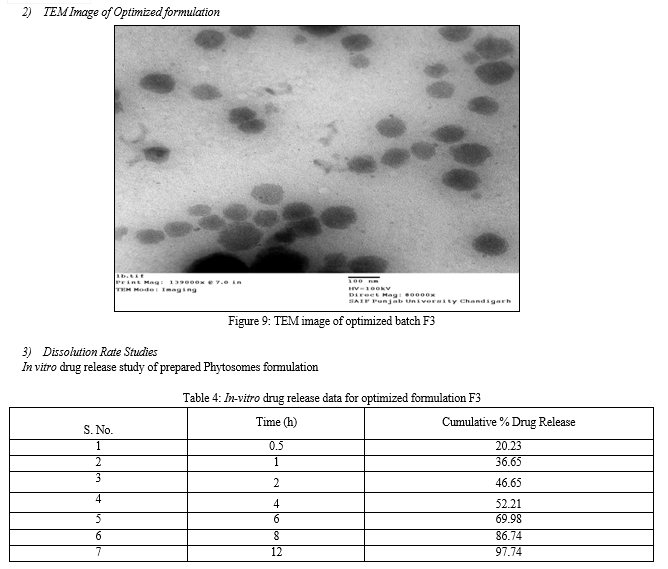
4. In Vitro dissoLution Rate Studies
The in vitro drug release of the prepared phytosomes was assessed using a USP-type I dissolution apparatus (basket type). The dissolution medium consisted of 900 mL of 0.1N hydrochloric acid and was placed in the dissolution flask. The temperature was maintained at 37 ± 0.5°C, and the apparatus was run at a speed of 75 rpm. Each basket of the dissolution apparatus contained 10 mg of the prepared phytosomes. The dissolution process was allowed to continue for a total of 8 hours. At specific intervals (30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, and 12 hours), samples were withdrawn by using a 10 mL pipette and measuring 3 mL of the sample. After each withdrawal, fresh dissolution medium (37°C) was replaced with the same quantity of the sample. The absorbance of each sample was measured at 274.0 nm using spectroscopy. This procedure was carried out for up to 12 hours, with the intention of assessing the release of the drug from the phytosomes over time. The use of 0.1N hydrochloric acid as the dissolution medium is a standard method for assessing drug release from oral formulations, and the USP-type I dissolution apparatus is commonly used for this purpose. The measured absorbance at 340.0 nm is a standard method for quantifying the drug concentration in the dissolution medium15.



IV. SUMMARY
The produced phytosome had distinct, spherical shape. Vesicles were found to have particles between 215-285 nm in size, where entrapment efficiency was discovered to be between 68% and 73%. Zeta potential of the optimised batch was discovered to be 36.45mv, indicating Phytosome stability. The value of drug release percentage is a sign of quick skin penetration that may be caused by vesicles with a nanometer-sized size. This is a positive discovery for extracts, which are poorly absorbed via the skin. Based on the results discussed above, it can be said that phytosomes containing Pterocarpus santalinus extract can serve as a practical and secure substitute for dosage forms.
References
[1] Zhang L, Demain AL. Natural Products. Drug Discov Ther Med p. 2005; [2] Guo Z. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7(2):119–36. [3] Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. [4] Bonati A. Formulation of plant extracts into dosage forms. In: The Medicinal Plant Industry. Routledge; 2017. p. 107–14. [5] Kumadoh D, Ofori-Kwakye K. Dosage forms of herbal medicinal products and their stability considerations-an overview. J Crit Rev. 2017;4(4):1–8. [6] Ajazuddin, Saraf S. Applications of novel drug delivery system for herbal formulations. Fitoterapia. 2010 Oct;81(7):680–9. [7] Azamthulla M, Rajkapoor B. A REVIEW ON PTEROCARPUS SANTALINUS LINN. 2015 Jan 1;4:282–92. [8] Bulle S, Reddyvari H, Nallanchakravarthula V, Vaddi DR. Therapeutic potential of Pterocarpus santalinus L.: an update. Pharmacogn Rev. 2016;10(19):43. [9] Sharma S, Roy RK. Phytosomes: an emerging technology. Int J Pharm Res Dev. 2010;2(5):1–7. [10] Patel J, Patel R, Khambholja K, Patel N. An overview of phytosomes as an advanced herbal drug delivery system. Asian J Pharm Sci. 2009;4(6):363–71 [11] Magdy IM, ?Optimization of chlorphenesin emulgel formulation?, AAPS J, 2004, 6(3), pp.1-7 [12] Sahu AR, Bothara SB. Formulation and evaluation of phytosome drug delivery system of boswellia serrata extract. Int J Res Med. 2015;4(2):94–9. [13] Patel R, Singh S, Navin S, R G. Development and Characterization of Curcumin Loaded Transfersome for Transdermal Delivery. J Pharm Sci Res. 2009 Jan 1(4), pp.71-80. [14] Sujit K.D, Sibaji S and Sumit C, ?Formulation and evaluation of aceclofenac gel?, Int. J.Chem. Tech. Res, 2009, 1(2), pp.204-207. [15] Chandira RM, Pradeep AP and Bhowmik D, ?Design development and formulation of antiacne dermatological gel?, J. Chem. Pharm. Res, 2010, 2(1), pp.401-414
Copyright
Copyright © 2024 Pooja Dhadse, Jyoti Saxena. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET57964
Publish Date : 2024-01-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

